Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O decomposition at the U
decomposition at the U O
O surface in bicarbonate solution
surface in bicarbonate solutionMcGrady, J.; Kumagai, Yuta; Watanabe, Masayuki; Kirishima, Akira*; Akiyama, Daisuke*; Kitamura, Akira; Kimuro, Shingo
RSC Advances (Internet), 11(46), p.28940 - 28948, 2021/08
Times Cited Count:5 Percentile:38.19(Chemistry, Multidisciplinary) -HF mixture; Kinetics and mechanism
-HF mixture; Kinetics and mechanismSimonnet, M.; Barr , N.*; Drot, R.*; Le Naour, C.*; Sladkov, V.*; Delpech, S.*
, N.*; Drot, R.*; Le Naour, C.*; Sladkov, V.*; Delpech, S.*
Radiochimica Acta, 107(4), p.289 - 297, 2019/04
Times Cited Count:3 Percentile:21.58(Chemistry, Inorganic & Nuclear)Wan, T.; Saito, Shigeru
Metals, 8(8), p.627_1 - 627_22, 2018/08
Times Cited Count:17 Percentile:66.2(Materials Science, Multidisciplinary) activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions
activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditionsSawaguchi, Takuma; Tsukada, Manabu; Yamaguchi, Tetsuji; Mukai, Masayuki
Clay Minerals, 51(2), p.267 - 278, 2016/05
Times Cited Count:7 Percentile:24.47(Chemistry, Physical)The dependences of the dissolution rate of compacted montmorillonite on activity of OH (a
(a -) and temperature (T) were investigated. The dissolution rate of montmorillonite (
-) and temperature (T) were investigated. The dissolution rate of montmorillonite (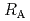 ) in compacted pure montmorillonite, which was formulized as
) in compacted pure montmorillonite, which was formulized as 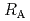 = 10
= 10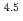 (a
(a -)
-)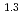 e
e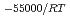 , was higher than that in the compacted sand-bentonite mixtures:
, was higher than that in the compacted sand-bentonite mixtures: 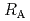 = 3500 (a
= 3500 (a -)
-)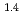 e
e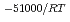 . The difference can be explained by considering the decrease in a
. The difference can be explained by considering the decrease in a - in the mixtures accompanied by dissolution of accessory minerals such as quartz and chalcedony. The dissolution rate model developed for pure montmorillonite is expected to be applied to bentonite mixtures if quantification of the decrease in a
- in the mixtures accompanied by dissolution of accessory minerals such as quartz and chalcedony. The dissolution rate model developed for pure montmorillonite is expected to be applied to bentonite mixtures if quantification of the decrease in a - is achieved somehow.
- is achieved somehow.
Kokusen, Junya; Seki, Masakazu; Abe, Masayuki; Nakazaki, Masato; Kida, Takashi; Umeda, Miki; Kihara, Takehiro; Sugikawa, Susumu
JAERI-Tech 2005-004, 53 Pages, 2005/03
This report presents operating records of dissolution of uranium dioxide and concentration of uranyl nitrate solution and acid removal, which have been performed from 1994 through 2003, for the purpose of feeding 10% and 6% enriched uranyl nitrate solution fuel to Static Experimental Critical Facility(STACY) and Transient Experimental Critical Facility(TRACY) in Nuclear Fuel Safety Engineering Facility(NUCEF).
Mineo, Hideaki; Isogai, Hikaru; Morita, Yasuji; Uchiyama, Gunzo*
Journal of Nuclear Science and Technology, 41(2), p.126 - 134, 2004/02
Times Cited Count:7 Percentile:45.11(Nuclear Science & Technology)A simple equation was proposed for the dissolution rate of spent LWR fuel, of which the change in the dissolution area was estimated by taking into account of the area of the cracks occurring due to thermal shrinkage of the pellets during irradiation. The applicability of proposed equation was examined using LWR fuel dissolution test results in the present study as well as the results obtained by other workers. The equation showed good agreements with the dissolution test results obtained from spent fuel pellets and pulverized spent fuel. It was indicated that the proposed equation was simple and would be useful for the prediction of dissolution of spent LWR fuels. However, the initial effective dissolution area, the parameter of the equation, was found to depend on the temperature, which could not be explained by the proposed equation. Further studies on the role of other factors affecting dissolution rate, such as nitrous acid, in the dissolution of spent fuel was required.
Mineo, Hideaki; Goto, Minoru; Iizuka, Masaru*; Fujisaki, Susumu; Hagiya, Hiromichi*; Uchiyama, Gunzo
Separation Science and Technology, 38(9), p.1981 - 2001, 2003/05
Times Cited Count:22 Percentile:63.74(Chemistry, Multidisciplinary)no abstracts in English
Umeda, Miki; Nakazaki, Masato; Kida, Takashi; Sato, Kenji; Kato, Tadahito; Kihara, Takehiro; Sugikawa, Susumu
JAERI-Tech 2003-024, 23 Pages, 2003/03
MOX dissolution with silver mediated electrolytic oxidation method is planned for the preparation of plutonium nitrate solution to be used for criticality safety experiments at Nuclear Fuel Cycle Safety Engineering Research Facility (NUCEF). Silver mediated electrolytic oxidation method uses the strong oxidisation ability of Ag(II) ion. This method is thought to be effective for the dissolution of MOX, which is difficult to be dissolved with nitric acid.In this paper, the results of experiments on dissolution with 100 g of MOX are described. It was confirmed by the results that the MOX powder to be used at NUCEF was completely dissolved by silver mediated electrolytic oxidation method and that Pu(VI) ion in the obtained solution was reduced to tetravalent by means of NO purging.
purging.
 O
O powders in nitric acid
powders in nitric acid;
Ind.Eng.Chem.,Process Des.Dev., 23, p.122 - 125, 1984/00
no abstracts in English